Volume 12, Issue 1 (10-2024)
Jorjani Biomed J 2024, 12(1): 23-27 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Eidizadeh H, Avandi S M, Zar A, Sadeghipour H R. The effect of eight weeks of resistance training with spirulina platensis supplementation on the RAGs/Rheb/mTORC/S6K pathway in male rat kidneys. Jorjani Biomed J 2024; 12 (1) :23-27
URL: http://goums.ac.ir/jorjanijournal/article-1-985-en.html
URL: http://goums.ac.ir/jorjanijournal/article-1-985-en.html
1- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,mohsenavandi@gmail.com
3- Department of Sport Science, School of Literature and Humanities, Persian Gulf University, Boushehr, Iran
2- Sport Science Department, Human Faculty, Semnan University, Semnan, Iran ,
3- Department of Sport Science, School of Literature and Humanities, Persian Gulf University, Boushehr, Iran
Keywords: Resistance training, Arthrospira platensis, kidney, mTORc1, RAGs, Rheb protein, rat, P70S6K
Full-Text [PDF 478 kb]
(921 Downloads)
| Abstract (HTML) (3558 Views)
Full-Text: (746 Views)
Introduction
The impact of supplements and exercise on health and safety has been relatively underexplored. Nevertheless, some studies have reported adverse effects of certain exercise supplements on kidney function (1). Spirulina, a blue-green alga that thrives in tropical and subtropical waters rich in carbonate and bicarbonate, boasts a high protein content ranging from 50% to 71% (2,3). Consumption of spirulina is known to reduce protein breakdown and enhance its synthesis, making it a recommended supplement for restoring body weight and muscle protein. Research indicates that combining spirulina with exercise significantly enhances isometric strength and endurance (4,5). Resistance training, a potent method for increasing muscle mass, is also a stimulus for altering homeostasis in skeletal muscle (6).
mTOR is a serine/threonine kinase that promotes protein synthesis and ribosome biogenesis while inhibiting a form of protein breakdown known as autophagy in some cells. Additionally, mTOR facilitates cell division and the transcription of certain genes (7,8). The phosphorylation of p70S6K, crucial for protein synthesis, occurs within the mTOR signaling pathway. p70S6K is a component of the mTORC1 signaling pathway (9). Various methods can activate mTORC1, but they all eventually activate mTOR through a small G-protein called Rheb (Ras homolog enriched in the brain). Rheb is believed to activate mTOR by recruiting phospholipase and increasing phosphatidic acid levels (8,10). mTOR regulates protein synthesis and cell growth by phosphorylating and thereby activating the 70 kDa ribosomal S6 kinase (p70S6K), which plays a regulatory role in protein synthesis and cell growth by phosphorylating its primary substrate, ribosomal protein S6, upon mitogen stimulation. Increased expression/activation of p70S6K is associated with poor prognosis in certain disease types, suggesting it may serve as a biomarker for some conditions (11).
Amino acids activate mTOR through small G-proteins called Rags. Since increased protein synthesis is a primary cause of hypertrophy and mTOR is central to protein synthesis, resistance training may induce hypertrophy by activating this pathway. It has been demonstrated that mTOR increases in response to resistance training (10). The mTOR signaling pathway plays a significant role in kidney tissue, contributing to the transformation of mesenchymal cells into epithelial cells and increasing the length of nephrons during development. This pathway is involved in various pathological processes in adulthood, including renal fibrosis from acute kidney injuries, renal cysts, and chronic kidney failure. Mice with deletions in some pathways of this signal at birth exhibit severe kidney defects (12). Exercise regulates the mTOR signaling pathway through several upstream mechanisms, and physical activity has been shown to activate some pathways in diabetic rats by increasing GSK-3β phosphorylation. Thus, exercise under diabetic conditions can influence the catenin-β/Wnt pathway by reducing GSK-3β gene expression, leading to decreased glucose levels and increased insulin levels in diabetic rats (13).
Pharmaceutical and dietary supplements can impact internal tissues, particularly the kidneys. For instance, protein supplements reportedly do not adversely affect kidney function (14). However, some studies suggest a relationship between dietary patterns and kidney function (15). It has been observed that a diet high in meat, seafood, and eggs negatively impacts kidney function, whereas diets rich in sausage, fruit, or dairy products improve kidney function (15). Additionally, improper creatine consumption can harm kidney function, although creatine taken with exercise results in less kidney damage than creatine taken alone (16). Research indicates that high-protein supplements combined with resistance training do not negatively affect kidney function. For example, a study demonstrated that high-protein intake with resistance training does not impair kidney function, although a high-protein diet alone affects the glomerular filtration rate and is linked to kidney damage (17).
Given that resistance training regulates protein synthesis and induces hypertrophy through mechanisms such as mTOR activation, and considering spirulina's high protein content and its ability to enhance protein synthesis, spirulina could potentially activate the mTOR pathway. Studies have shown that activating the mTOR pathway and increasing its expression can exacerbate kidney disease (18). Recent studies have emphasized the importance of mTOR activation in cell proliferation and cyst growth in polycystic kidney disease. Abnormal regulation of mTOR, phosphorylated S6K (P-S6K), and phosphorylated S6 (P-S6) levels has been observed in the renal cysts of rats with autosomal dominant polycystic kidney disease (ADPKD) (19). Inhibition of mTOR and related signals reduced cyst growth and kidney enlargement, preserving kidney function in rats (19). Despite previous studies not addressing the effect of spirulina supplements on the mTOR pathway affecting kidney tissue, and ambiguous results regarding the impact of physical exercise, this study aims to evaluate the effect of eight weeks of resistance training with Spirulina platensis supplementation on the RAGs/Rheb/mTORC1/S6K pathway in the kidney tissue of soft rats.
Methods
Study design
The statistical population of this study consisted of male Sprague-Dawley rats. Thirty-two young male rats, aged 3 months with a mean weight of 290 ± 20 g, were selected. Throughout the study period, each rat was housed in a separate transparent polycarbonate cage in an environment maintained at a temperature of 22 ± 2°C, humidity of 55 ± 4%, and a 12:12 hour light-dark cycle. All animals had free access to standard rat food (Rat pellets prepared by Behparvar Company) and safe water and were cared for in accordance with relevant guidelines. The rats were grouped and, after the training period, were sacrificed to measure the desired factors.
Experimental animal groups
Initially, the rats were kept in the laboratory for a week to adapt to the environment. After one week of adaptation, the rats were introduced to resistance training and climbing the ladder once a day for 3 days. The ladder used was one meter high with a perpendicular slope to the ground, and the distance between steps was 4 cm. To encourage the rats to climb, their tails were gently touched to prompt movement. The introductory program involved three sessions of ladder climbing, each session comprising three to four repetitions without weights, conducted over one week. Following the introduction phase, the rats were randomly divided into the following four groups (8 rats in each group):
Group A: Control group (No resistance training, no spirulina supplementation)
Group B: Supplement group (No resistance training, spirulina supplement)
Group C: Resistance training group (No spirulina supplement)
Group D: Resistance training + Supplement group (Spirulina supplement)
Resistance training protocol
The resistance training protocol consisted of eight weeks of climbing a resistance training ladder. Each session began with a warm-up involving three repetitions without weights, with rest intervals between repetitions. The initial weight was set at 30% of the rats' body weight, gradually increasing to 100% by the final week. At the beginning of each week, the average weight of each group was measured, and weights were selected accordingly. During training sessions, weights were attached to the rats' tails using leucoplast adhesive. The training load consisted of 50%, 75%, 90%, and 100% of the selected weight for that week. Each training session included 3 sets of 5 repetitions with 1-minute rest between repetitions and 2 minutes rest between sets.
Spirulina supplementation
Spirulina was added to the drinking water of rats in the spirulina supplement groups (Groups B and D) at a dose of 200 mg/kg/day, starting 24 hours before the study and continued daily until the end of the eighth week.
Sampling
Twenty-four hours after the final training session, all rats were anesthetized with an injection of ketamine 10% (50 mg/kg body weight) and xylazine 2% (10 mg/kg body weight) for about five minutes and then decapitated. The kidneys were immediately removed, placed in nitrogen tanks, and transferred to an 80-degree freezer for RNA extraction. RT-PCR was used to measure the expression of Rheb, RAGs, mTOR, and p70S6K genes.
Statistical analysis
The Shapiro-Wilk test was used to assess the distribution of data within the research groups. The assumption of normality was confirmed, and a two-way ANOVA (SPSS version 18) was employed to examine the effects of interventions at a significance level of P < 0.05.
Results
Animal body weight and Rheb, RAGs, mTOR, p70S6K
Changes in animal weight and the expression levels of Rheb, RAGs, mTOR, and p70S6K in the Spirulina (SP), Resistance Exercise (RE), Spirulina + Resistance Exercise (SP + RE), and control (CO) groups are shown in Table 1. The results indicate that, after eight weeks, the body weight of rats increased in all groups (CO, SP, RE, and SP + RE).
mTOR changes
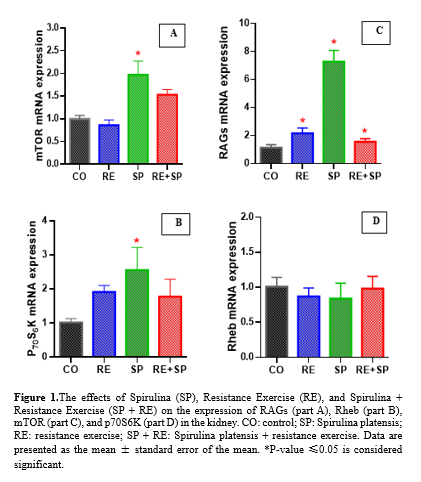
The expression of mTOR changed significantly across the groups by the end of the study period. Specifically, mTOR expression increased significantly in the SP group (p-value = 0.01), decreased non-significantly in the RE group (p-value = 0.16), and increased non-significantly in the RE + SP group (p-value = 0.06) (Table 2; Fig. 1A).
p70S6K changes
The expression of p70S6K also varied among the groups. There was a significant increase in p70S6K expression in the SP group (p-value = 0.044), while the changes in the RE + SP group (p-value = 0.37) and the RE group (p-value = 0.16) were not significant (Table 2; Fig. 1B).
RAGs changes
The expression of RAGs significantly increased in all experimental groups. The RE group (p-value = 0.047), the RE + SP group (p-value = 0.025), and the SP group (p-value = 0.001) all showed significant increases in RAGs expression (Table 2; Fig. 1C).
Rheb changes
The expression of Rheb did not change significantly in any of the groups by the end of the study period. The expression levels in the RE group (p-value = 0.13), SP + RE group (p-value = 0.70), and SP group (p-value = 0.28) were not significant (Table 2; Fig. 1D).
Discussion
The aim of this study was to investigate the effect of eight weeks of resistance training with Spirulina platensis supplementation on the RAGs/Rheb/mTORC/p70S6K pathway in male rats. The main findings indicate that mTOR gene expression significantly increased only in the supplement group. In contrast, significant changes in RAGs gene expression were observed in the supplementation group, resistance training group, and the training + supplementation group. However, Rheb gene expression changes were not significant in any of the groups, and significant changes in p70S6K gene expression were observed only in the supplement group.
The significant increase in mTOR gene expression in the spirulina supplement group, but not in the resistance training or training + supplementation groups, contrasts with some previous studies. For example, Nemati et al. (2016) (20) and Sharafati Moghadam et al. (2018) (21) reported significant increases in mTOR gene expression in the skeletal muscles of diabetic rats. Conversely, Haraguchi et al. (2013) (22) found that resistance training did not significantly change mTOR gene expression in rats. This discrepancy might be due to the influence of regulated in development and DNA damage response 1 (REDD1) on the mTOR protein synthesis pathway. Increased REDD1 protein expression can decrease mTOR gene expression (23). Additionally, low-intensity physical activity can increase negative regulators of protein synthesis such as AMPK and 4E-BP1, with AMPK preventing mTOR activation by activating the TSC1/2 complex (24). Luciano et al. (2017) demonstrated that high-intensity hypertrophy resistance training was more effective than low-intensity endurance training in activating mTOR (25). The intensity of the exercise program in this study may not have been sufficient to significantly increase mTOR gene expression.
Nutrition, particularly protein intake, can increase serum amino acid levels, which are crucial for mTOR activation (26). Spirulina, rich in amino acids and antioxidants, appears to be a potent activator of mTOR and the autophagy process. The significant increase in mTOR gene expression observed only in the spirulina supplement group suggests that the supplement dosage might not be suitable for diabetic patients. Spirulina contains essential amino acids, including leucine, which can stimulate mTOR activation (27). Amino acids activate mTOR through RAGs independently of other activators. In this study, resistance training and resistance training with spirulina supplementation did not significantly increase mTOR gene expression, likely due to insufficient Rheb activation. Rheb is crucial for S6K and mTOR communication, and mTOR inhibitors can disrupt this communication through TSC activation (28). Many inhibitors, such as AMPK, can interrupt this communication (24). Therefore, while spirulina supplementation alone may be harmful to kidney patients, combining it with resistance training may mitigate this risk, as a significant increase in mTOR was not observed in the training or combined groups.
RAGs gene expression significantly changed in all experimental groups, consistent with the findings of Kim et al. (2008) and Sancka et al. (2008) (29,30). Kim et al. (2008) reported that in mammalian cells, constitutively active (GTP-bound) Rag activated TORC1 in the presence of amino acids, suggesting that spirulina's amino acid content led to increased RAGs expression. However, Rheb gene expression did not significantly change in any of the research groups, contrasting with Song et al. (2017) (31). Resistance training maintains or improves cell survival by activating growth factors, which in turn activate PI3K and AKT, regulating cell function through downstream proteins including mTOR (32). Two small GTPases, Rheb and Rags, activate mTOR in response to growth factors and amino acids, respectively (33). In this study, spirulina's amino acids likely increased RAGs expression, leading to mTOR activation in the supplement group. However, the inability to significantly increase mTOR gene expression in the resistance training and training + supplementation groups suggests that Rheb activation was insufficient. This finding is inconsistent with Ma and Blenis (2009), who identified Rheb as the primary factor in mTOR activation, as mTOR increased significantly in the spirulina group without a significant increase in Rheb (34). Increased mTOR activity is known to contribute to kidney tubular damage (33). The activation of this gene following supplementation might not yield positive outcomes, necessitating further studies with different dosages. The significant increase in mTOR and RAGs expression in the spirulina group, potentially harmful in kidney diseases such as polycystic kidney disease and nephropathy, requires careful consideration. However, the combined use of spirulina and resistance training appears less harmful, as indicated by the lack of significant changes in both mTOR and RAGs expressions. Nonetheless, research has indicated that in humans, 70S6K can be activated by Akt-independent pathways, and mTOR can be activated through various signaling mechanisms. Therefore, a comprehensive understanding of these processes in kidney tissue necessitates additional research (35). Given the role of S6K in the downstream pathway of mTOR signaling, its significant increase in the supplement group alone indicates that spirulina supplementation may be dangerous for kidney patients without the mitigating effect of resistance training.
The significant increase in p70S6K gene expression only in the spirulina supplement group aligns with findings from Sadeghipour et al. (2024), who reported significant increases in S6K gene expression following combined resistance training and spirulina supplementation (36). Mirzaei et al. (2023) observed significant decreases in S6K1 protein content in cardiac tissue after a 2-week resistance and aerobic training program, but gene expression analysis showed significant increases in S6K1 gene expression (37). Mousavi Muzafar (2020) reported significant differences in S6K levels across all three groups in a study on the effects of beta-hydroxy beta-methylbutyrate (HMB) supplementation and resistance training (38). The present study's findings contrast with those of Dreyer, Fujita et al. (2010) (39) and Hulmi, Tannerstedt et al. (2009) (40), but Haraguchi et al. (2014) observed increased mTOR expression with whey protein consumption, while exercise reduced mTOR expression (22). Amiri et al. (2019) found that HMB supplementation decreased myostatin and increased S6K gene levels, with greater effects when combined with resistance training (41). In this study, significant increases in mTOR and S6K gene expression in the supplement group suggest that amino acids in spirulina activate S6K through an mTOR-independent pathway, potentially involving PDK1 and PIF (27). Recent studies indicate that resistance activity can activate S6K through an AKT-independent pathway (42).
Therefore, while spirulina supplementation alone may increase the expression of mTOR and S6K, potentially harmful to kidney tissue, combining it with resistance training may mitigate this risk. The increase in S6K gene expression in the supplement group indicates that spirulina supplementation alone might be dangerous for patients with polycystic kidney disease and nephropathy. However, further investigations are necessary to understand the impact of spirulina supplementation with resistance training on kidney tissue. The inability to control daily activities of the rats, stress on the rats, and lack of protein content evaluation of the investigated variables are limitations of the present study.
Conclusion
Based on the findings of this study, eight weeks of resistance training did not significantly affect the mTOR pathway. However, spirulina supplementation had a significant effect, increasing mTOR pathway activity. Notably, there was no significant increase in this pathway in the group that combined exercise with spirulina supplementation. The results indicated higher expression levels of mTOR, RAGs, and S6K genes in the spirulina group. Given that RAGs mediate the activation of the mTOR pathway via amino acids and considering the role of mTOR and S6K in the development of kidney diseases such as polycystic kidney disease and diabetic nephropathy, it is not recommended for this group of patients to use spirulina alone. However, since no significant increase in mTOR was observed in the combined exercise and spirulina group, it is possible that the benefits of spirulina could be harnessed in conjunction with resistance training without posing a risk to kidney patients. This hypothesis requires further investigation in human samples.
Acknowledgement
We are grateful to all coworkers involved in this study.
Funding sources
This study did not receive any funding.
Ethical statement
The study was approved by the Ethics Committee of Jahrom University of Medical Sciences and was carried out in strict accordance with the United States Institute of Animal Research guidelines for the care and use of laboratory animals (IR.JUMS.REC.1398.011).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
All authors equally contributed to the writing and revision of this paper.
The impact of supplements and exercise on health and safety has been relatively underexplored. Nevertheless, some studies have reported adverse effects of certain exercise supplements on kidney function (1). Spirulina, a blue-green alga that thrives in tropical and subtropical waters rich in carbonate and bicarbonate, boasts a high protein content ranging from 50% to 71% (2,3). Consumption of spirulina is known to reduce protein breakdown and enhance its synthesis, making it a recommended supplement for restoring body weight and muscle protein. Research indicates that combining spirulina with exercise significantly enhances isometric strength and endurance (4,5). Resistance training, a potent method for increasing muscle mass, is also a stimulus for altering homeostasis in skeletal muscle (6).
mTOR is a serine/threonine kinase that promotes protein synthesis and ribosome biogenesis while inhibiting a form of protein breakdown known as autophagy in some cells. Additionally, mTOR facilitates cell division and the transcription of certain genes (7,8). The phosphorylation of p70S6K, crucial for protein synthesis, occurs within the mTOR signaling pathway. p70S6K is a component of the mTORC1 signaling pathway (9). Various methods can activate mTORC1, but they all eventually activate mTOR through a small G-protein called Rheb (Ras homolog enriched in the brain). Rheb is believed to activate mTOR by recruiting phospholipase and increasing phosphatidic acid levels (8,10). mTOR regulates protein synthesis and cell growth by phosphorylating and thereby activating the 70 kDa ribosomal S6 kinase (p70S6K), which plays a regulatory role in protein synthesis and cell growth by phosphorylating its primary substrate, ribosomal protein S6, upon mitogen stimulation. Increased expression/activation of p70S6K is associated with poor prognosis in certain disease types, suggesting it may serve as a biomarker for some conditions (11).
Amino acids activate mTOR through small G-proteins called Rags. Since increased protein synthesis is a primary cause of hypertrophy and mTOR is central to protein synthesis, resistance training may induce hypertrophy by activating this pathway. It has been demonstrated that mTOR increases in response to resistance training (10). The mTOR signaling pathway plays a significant role in kidney tissue, contributing to the transformation of mesenchymal cells into epithelial cells and increasing the length of nephrons during development. This pathway is involved in various pathological processes in adulthood, including renal fibrosis from acute kidney injuries, renal cysts, and chronic kidney failure. Mice with deletions in some pathways of this signal at birth exhibit severe kidney defects (12). Exercise regulates the mTOR signaling pathway through several upstream mechanisms, and physical activity has been shown to activate some pathways in diabetic rats by increasing GSK-3β phosphorylation. Thus, exercise under diabetic conditions can influence the catenin-β/Wnt pathway by reducing GSK-3β gene expression, leading to decreased glucose levels and increased insulin levels in diabetic rats (13).
Pharmaceutical and dietary supplements can impact internal tissues, particularly the kidneys. For instance, protein supplements reportedly do not adversely affect kidney function (14). However, some studies suggest a relationship between dietary patterns and kidney function (15). It has been observed that a diet high in meat, seafood, and eggs negatively impacts kidney function, whereas diets rich in sausage, fruit, or dairy products improve kidney function (15). Additionally, improper creatine consumption can harm kidney function, although creatine taken with exercise results in less kidney damage than creatine taken alone (16). Research indicates that high-protein supplements combined with resistance training do not negatively affect kidney function. For example, a study demonstrated that high-protein intake with resistance training does not impair kidney function, although a high-protein diet alone affects the glomerular filtration rate and is linked to kidney damage (17).
Given that resistance training regulates protein synthesis and induces hypertrophy through mechanisms such as mTOR activation, and considering spirulina's high protein content and its ability to enhance protein synthesis, spirulina could potentially activate the mTOR pathway. Studies have shown that activating the mTOR pathway and increasing its expression can exacerbate kidney disease (18). Recent studies have emphasized the importance of mTOR activation in cell proliferation and cyst growth in polycystic kidney disease. Abnormal regulation of mTOR, phosphorylated S6K (P-S6K), and phosphorylated S6 (P-S6) levels has been observed in the renal cysts of rats with autosomal dominant polycystic kidney disease (ADPKD) (19). Inhibition of mTOR and related signals reduced cyst growth and kidney enlargement, preserving kidney function in rats (19). Despite previous studies not addressing the effect of spirulina supplements on the mTOR pathway affecting kidney tissue, and ambiguous results regarding the impact of physical exercise, this study aims to evaluate the effect of eight weeks of resistance training with Spirulina platensis supplementation on the RAGs/Rheb/mTORC1/S6K pathway in the kidney tissue of soft rats.
Methods
Study design
The statistical population of this study consisted of male Sprague-Dawley rats. Thirty-two young male rats, aged 3 months with a mean weight of 290 ± 20 g, were selected. Throughout the study period, each rat was housed in a separate transparent polycarbonate cage in an environment maintained at a temperature of 22 ± 2°C, humidity of 55 ± 4%, and a 12:12 hour light-dark cycle. All animals had free access to standard rat food (Rat pellets prepared by Behparvar Company) and safe water and were cared for in accordance with relevant guidelines. The rats were grouped and, after the training period, were sacrificed to measure the desired factors.
Experimental animal groups
Initially, the rats were kept in the laboratory for a week to adapt to the environment. After one week of adaptation, the rats were introduced to resistance training and climbing the ladder once a day for 3 days. The ladder used was one meter high with a perpendicular slope to the ground, and the distance between steps was 4 cm. To encourage the rats to climb, their tails were gently touched to prompt movement. The introductory program involved three sessions of ladder climbing, each session comprising three to four repetitions without weights, conducted over one week. Following the introduction phase, the rats were randomly divided into the following four groups (8 rats in each group):
Group A: Control group (No resistance training, no spirulina supplementation)
Group B: Supplement group (No resistance training, spirulina supplement)
Group C: Resistance training group (No spirulina supplement)
Group D: Resistance training + Supplement group (Spirulina supplement)
Resistance training protocol
The resistance training protocol consisted of eight weeks of climbing a resistance training ladder. Each session began with a warm-up involving three repetitions without weights, with rest intervals between repetitions. The initial weight was set at 30% of the rats' body weight, gradually increasing to 100% by the final week. At the beginning of each week, the average weight of each group was measured, and weights were selected accordingly. During training sessions, weights were attached to the rats' tails using leucoplast adhesive. The training load consisted of 50%, 75%, 90%, and 100% of the selected weight for that week. Each training session included 3 sets of 5 repetitions with 1-minute rest between repetitions and 2 minutes rest between sets.
Spirulina supplementation
Spirulina was added to the drinking water of rats in the spirulina supplement groups (Groups B and D) at a dose of 200 mg/kg/day, starting 24 hours before the study and continued daily until the end of the eighth week.
Sampling
Twenty-four hours after the final training session, all rats were anesthetized with an injection of ketamine 10% (50 mg/kg body weight) and xylazine 2% (10 mg/kg body weight) for about five minutes and then decapitated. The kidneys were immediately removed, placed in nitrogen tanks, and transferred to an 80-degree freezer for RNA extraction. RT-PCR was used to measure the expression of Rheb, RAGs, mTOR, and p70S6K genes.
Statistical analysis
The Shapiro-Wilk test was used to assess the distribution of data within the research groups. The assumption of normality was confirmed, and a two-way ANOVA (SPSS version 18) was employed to examine the effects of interventions at a significance level of P < 0.05.
Results
Animal body weight and Rheb, RAGs, mTOR, p70S6K
Changes in animal weight and the expression levels of Rheb, RAGs, mTOR, and p70S6K in the Spirulina (SP), Resistance Exercise (RE), Spirulina + Resistance Exercise (SP + RE), and control (CO) groups are shown in Table 1. The results indicate that, after eight weeks, the body weight of rats increased in all groups (CO, SP, RE, and SP + RE).
mTOR changes
The expression of mTOR changed significantly across the groups by the end of the study period. Specifically, mTOR expression increased significantly in the SP group (p-value = 0.01), decreased non-significantly in the RE group (p-value = 0.16), and increased non-significantly in the RE + SP group (p-value = 0.06) (Table 2; Fig. 1A).
p70S6K changes
The expression of p70S6K also varied among the groups. There was a significant increase in p70S6K expression in the SP group (p-value = 0.044), while the changes in the RE + SP group (p-value = 0.37) and the RE group (p-value = 0.16) were not significant (Table 2; Fig. 1B).
RAGs changes
The expression of RAGs significantly increased in all experimental groups. The RE group (p-value = 0.047), the RE + SP group (p-value = 0.025), and the SP group (p-value = 0.001) all showed significant increases in RAGs expression (Table 2; Fig. 1C).
Rheb changes
The expression of Rheb did not change significantly in any of the groups by the end of the study period. The expression levels in the RE group (p-value = 0.13), SP + RE group (p-value = 0.70), and SP group (p-value = 0.28) were not significant (Table 2; Fig. 1D).
|
Table 1. Comparison of the effects of spirulina, resistance exercise, or spirulina + resistance exercise on changes in rat weight and expression levels of Rheb, RAGs, mTOR, and p70S6K after the experimental study
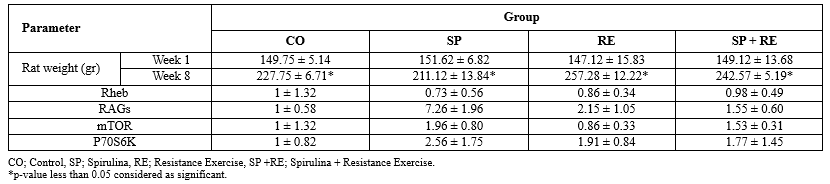 Table 2. Results of two-way analysis of variance to evaluate the effects of training and consumption of spirulina on Rheb, RAGs, mTOR, p70S6K in the kidney tissues of male rats 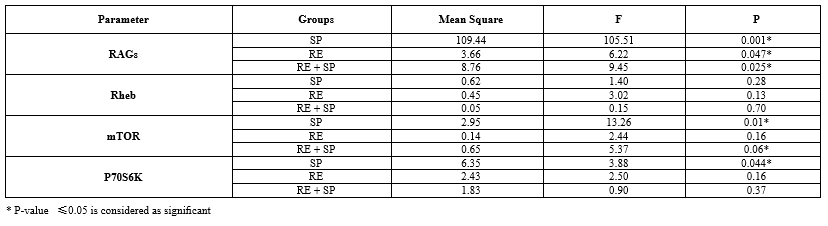 |

Discussion
The aim of this study was to investigate the effect of eight weeks of resistance training with Spirulina platensis supplementation on the RAGs/Rheb/mTORC/p70S6K pathway in male rats. The main findings indicate that mTOR gene expression significantly increased only in the supplement group. In contrast, significant changes in RAGs gene expression were observed in the supplementation group, resistance training group, and the training + supplementation group. However, Rheb gene expression changes were not significant in any of the groups, and significant changes in p70S6K gene expression were observed only in the supplement group.
The significant increase in mTOR gene expression in the spirulina supplement group, but not in the resistance training or training + supplementation groups, contrasts with some previous studies. For example, Nemati et al. (2016) (20) and Sharafati Moghadam et al. (2018) (21) reported significant increases in mTOR gene expression in the skeletal muscles of diabetic rats. Conversely, Haraguchi et al. (2013) (22) found that resistance training did not significantly change mTOR gene expression in rats. This discrepancy might be due to the influence of regulated in development and DNA damage response 1 (REDD1) on the mTOR protein synthesis pathway. Increased REDD1 protein expression can decrease mTOR gene expression (23). Additionally, low-intensity physical activity can increase negative regulators of protein synthesis such as AMPK and 4E-BP1, with AMPK preventing mTOR activation by activating the TSC1/2 complex (24). Luciano et al. (2017) demonstrated that high-intensity hypertrophy resistance training was more effective than low-intensity endurance training in activating mTOR (25). The intensity of the exercise program in this study may not have been sufficient to significantly increase mTOR gene expression.
Nutrition, particularly protein intake, can increase serum amino acid levels, which are crucial for mTOR activation (26). Spirulina, rich in amino acids and antioxidants, appears to be a potent activator of mTOR and the autophagy process. The significant increase in mTOR gene expression observed only in the spirulina supplement group suggests that the supplement dosage might not be suitable for diabetic patients. Spirulina contains essential amino acids, including leucine, which can stimulate mTOR activation (27). Amino acids activate mTOR through RAGs independently of other activators. In this study, resistance training and resistance training with spirulina supplementation did not significantly increase mTOR gene expression, likely due to insufficient Rheb activation. Rheb is crucial for S6K and mTOR communication, and mTOR inhibitors can disrupt this communication through TSC activation (28). Many inhibitors, such as AMPK, can interrupt this communication (24). Therefore, while spirulina supplementation alone may be harmful to kidney patients, combining it with resistance training may mitigate this risk, as a significant increase in mTOR was not observed in the training or combined groups.
RAGs gene expression significantly changed in all experimental groups, consistent with the findings of Kim et al. (2008) and Sancka et al. (2008) (29,30). Kim et al. (2008) reported that in mammalian cells, constitutively active (GTP-bound) Rag activated TORC1 in the presence of amino acids, suggesting that spirulina's amino acid content led to increased RAGs expression. However, Rheb gene expression did not significantly change in any of the research groups, contrasting with Song et al. (2017) (31). Resistance training maintains or improves cell survival by activating growth factors, which in turn activate PI3K and AKT, regulating cell function through downstream proteins including mTOR (32). Two small GTPases, Rheb and Rags, activate mTOR in response to growth factors and amino acids, respectively (33). In this study, spirulina's amino acids likely increased RAGs expression, leading to mTOR activation in the supplement group. However, the inability to significantly increase mTOR gene expression in the resistance training and training + supplementation groups suggests that Rheb activation was insufficient. This finding is inconsistent with Ma and Blenis (2009), who identified Rheb as the primary factor in mTOR activation, as mTOR increased significantly in the spirulina group without a significant increase in Rheb (34). Increased mTOR activity is known to contribute to kidney tubular damage (33). The activation of this gene following supplementation might not yield positive outcomes, necessitating further studies with different dosages. The significant increase in mTOR and RAGs expression in the spirulina group, potentially harmful in kidney diseases such as polycystic kidney disease and nephropathy, requires careful consideration. However, the combined use of spirulina and resistance training appears less harmful, as indicated by the lack of significant changes in both mTOR and RAGs expressions. Nonetheless, research has indicated that in humans, 70S6K can be activated by Akt-independent pathways, and mTOR can be activated through various signaling mechanisms. Therefore, a comprehensive understanding of these processes in kidney tissue necessitates additional research (35). Given the role of S6K in the downstream pathway of mTOR signaling, its significant increase in the supplement group alone indicates that spirulina supplementation may be dangerous for kidney patients without the mitigating effect of resistance training.
The significant increase in p70S6K gene expression only in the spirulina supplement group aligns with findings from Sadeghipour et al. (2024), who reported significant increases in S6K gene expression following combined resistance training and spirulina supplementation (36). Mirzaei et al. (2023) observed significant decreases in S6K1 protein content in cardiac tissue after a 2-week resistance and aerobic training program, but gene expression analysis showed significant increases in S6K1 gene expression (37). Mousavi Muzafar (2020) reported significant differences in S6K levels across all three groups in a study on the effects of beta-hydroxy beta-methylbutyrate (HMB) supplementation and resistance training (38). The present study's findings contrast with those of Dreyer, Fujita et al. (2010) (39) and Hulmi, Tannerstedt et al. (2009) (40), but Haraguchi et al. (2014) observed increased mTOR expression with whey protein consumption, while exercise reduced mTOR expression (22). Amiri et al. (2019) found that HMB supplementation decreased myostatin and increased S6K gene levels, with greater effects when combined with resistance training (41). In this study, significant increases in mTOR and S6K gene expression in the supplement group suggest that amino acids in spirulina activate S6K through an mTOR-independent pathway, potentially involving PDK1 and PIF (27). Recent studies indicate that resistance activity can activate S6K through an AKT-independent pathway (42).
Therefore, while spirulina supplementation alone may increase the expression of mTOR and S6K, potentially harmful to kidney tissue, combining it with resistance training may mitigate this risk. The increase in S6K gene expression in the supplement group indicates that spirulina supplementation alone might be dangerous for patients with polycystic kidney disease and nephropathy. However, further investigations are necessary to understand the impact of spirulina supplementation with resistance training on kidney tissue. The inability to control daily activities of the rats, stress on the rats, and lack of protein content evaluation of the investigated variables are limitations of the present study.
Conclusion
Based on the findings of this study, eight weeks of resistance training did not significantly affect the mTOR pathway. However, spirulina supplementation had a significant effect, increasing mTOR pathway activity. Notably, there was no significant increase in this pathway in the group that combined exercise with spirulina supplementation. The results indicated higher expression levels of mTOR, RAGs, and S6K genes in the spirulina group. Given that RAGs mediate the activation of the mTOR pathway via amino acids and considering the role of mTOR and S6K in the development of kidney diseases such as polycystic kidney disease and diabetic nephropathy, it is not recommended for this group of patients to use spirulina alone. However, since no significant increase in mTOR was observed in the combined exercise and spirulina group, it is possible that the benefits of spirulina could be harnessed in conjunction with resistance training without posing a risk to kidney patients. This hypothesis requires further investigation in human samples.
Acknowledgement
We are grateful to all coworkers involved in this study.
Funding sources
This study did not receive any funding.
Ethical statement
The study was approved by the Ethics Committee of Jahrom University of Medical Sciences and was carried out in strict accordance with the United States Institute of Animal Research guidelines for the care and use of laboratory animals (IR.JUMS.REC.1398.011).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
All authors equally contributed to the writing and revision of this paper.
Type of Article: Original article |
Subject:
Health
Received: 2023/09/2 | Accepted: 2024/03/9 | Published: 2024/03/20
Received: 2023/09/2 | Accepted: 2024/03/9 | Published: 2024/03/20
References
1. Souza RA, Miranda H, Xavier M, Lazo-Osorio RA, Gouvea HA, Cogo JC, et al. Effects of high-dose creatine supplementation on kidney and liver responses in sedentary and exercised rats. J Sports Sci Med. 2009;8(4):672-81. [View at Publisher] [PMID] [Google Scholar]
2. Kuhn MA, Winston D. Herbal therapy and supplements: a scientific and traditional approach. Philadelphia:Lippincott Williams & Wilkins;2000. [View at Publisher] [Google Scholar]
3. Ali SK, Saleh AM . Spirulina-an overview. Int J Pharm Pharm Sci. 2012;4(3):9-15. [View at Publisher] [Google Scholar]
4. Sandhu J, Dheera B, Shweta S. Efficacy of spirulina supplementation on isometric strength and isometric endurance of quadriceps in trained and untrained individuals-a comparative study. Ibnosina Journal of Medicine and Biomedical Sciences. 2010;2(02):79-86. [View at Publisher] [DOI] [Google Scholar]
5. Gutiérrez-Salmeán G, Fabila-Castillo L, Chamorro-Cevallos G. Aspectos nutricionales y toxicológicos de Spirulina (arthrospira). Nutr Hosp. 2015;32(1):34-40. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Adegoke OA, Abdullahi A, Tavajohi-Fini P. mTORC1 and the regulation of skeletal muscle anabolism and mass. Appl Physiol Nutr Metab. 2012;37(3):395-406. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Wackerhage H, Smith J, Wisneiwski D. Molecular exercise physiology; Oxford textbook of childrenVs sport and exercise medicine. Oxford;Oxford University Press:2017. p.429-440. [View at Publisher] [DOI] [Google Scholar]
9. Sengupta S, Peterson TR , Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010:40(2):310-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, et al. The role of skeletal muscle mTOR in the regulation of mechanical load‐induced growth. J Physiol. 2011;589(22):5485-501. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Androulakis-Korakakis P, Fisher JP, Steele J. The minimum effective training dose required to increase 1RM strength in resistance-trained men: a systematic review and meta-analysis. Sports Med. 2020;50(4):751-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling-a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17(3):172-84. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Daneshmandi H, Azamian Jazi A, Ghasemi B. Effects of Eight Weeks of Moderate-Intensity Continuous and Resistance Training on The Glycogen Synthase Kinase-3 Beta Gene Expression, and Serum Glucose and Insulin Levels in Streptozotocin-Induced Diabetic Rats. Journal of Advanced Biomedical Sciences. 2020;10(4):2716-27. [View at Publisher] [DOI] [Google Scholar]
14. Antonio J, Ellerbroek A, Silver T, Vargas L, Tamayo A, Buehn R, et al. A high protein diet has no harmful effects: a one-year crossover study in resistance-trained males. J Nutr Metab. 2016;2016:9104792. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Syauqy A, Hsu CY, Lee HA, Rau HH, Chao JCJ. Association between Dietary Patterns and Kidney Function Parameters in Adults with Metabolic Syndrome: A Cross-Sectional Study. Nutrients. 2021;13(1):40. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Lugaresi R, Leme M, Painelli VS, Murai IH, Roschel H, Sapienza MT, et al. Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet? J Int Soc Sports Nutr. 2013;10(1):26. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Singer MA. Dietary protein-induced changes in excretory function: a general animal design feature. Comp Biochem Physiol B Biochem Mol Biol. 2003;136(4):785-801. [View at Publisher] [DOI] [PMID] [Google Scholar]
18. Grahammer F, Wanner N, Huber TB. mTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant. 2014;29(suppl1):i9-18. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Leonhard WN, Song X, Kanhai AA, Iliuta IA, Bozovic A, Steinberg GR, et al. Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. EBioMedicine. 2019;47:436-445. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Nemati J, Samadi M, Hadidi V, MACINTASH B. The effect of performing a resistance training course on mTOR and p70s6k signaling pathway in skeletal muscle of male rats. Journal of Sport and Exercise Physiology. 2015;8(1):1149-56. [View at Publisher] [Google Scholar]
21. SherafatiMoghadam M, Salesi M, Daryanoosh F, Hemati Nafar M, Fallahi A. The effect of 4 weeks of high intensity interval training on the content of AKT1, mTOR, P70S6K1 and 4E-BP1 in soleus skeletal muscle of rats with type 2 diabetes: An experimental study. J Rafsanjan Univ Med Sci. 2018;17(9):843-54. [View at Publisher] [DOI] [Google Scholar]
22. Haraguchi FK, Magalhães CLB, Neves LX, Santos RC, Pedrosa ML, Silva ME. Whey protein modifies gene expression related to protein metabolism affecting muscle weight in resistance-exercised rats. Nutrition. 2014;30(7-8):876-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Gordon BS, Williamson DL, Lang CH, Jefferson LS, Kimball SR. Nutrient-induced stimulation of protein synthesis in mouse skeletal muscle is limited by the mTORC1 repressor REDD1. J Nutr. 2015;145(4):708-713. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Rose AJ, Bisiani B, Vistisen B, Kiens B, Richter EA. Skeletal muscle eEF2 and 4EBP1 phosphorylation during endurance exercise is dependent on intensity and muscle fiber type. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R326-33. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Luciano TF, Marques SO, Pieri BL, Souza DR, Araújo LV, Nesi RT, et al. Responses of skeletal muscle hypertrophy in Wistar rats to different resistance exercise models. Physiol Res. 2017;66(2):317-23. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Ahmadi F, Ghanbar Zadeh M, Habibi AH, Karimi F. Effect of resistance training with Spirulina platensis on PI3K/Akt/mTOR/p70S6k signaling pathway in cardiac muscle. Science & Sports. 2020;35(2):91-8. [View at Publisher] [DOI] [Google Scholar]
27. Sanchez Canedo C, Demeulder B, Ginion A, Bayascas JR, Balligand JL, Alessi DR, et al. Activation of the cardiac mTOR/p70S6K pathway by leucine requires PDK1 and correlates with PRAS40 phosphorylation. Am J Physiol Endocrinol Metab. 2010;298(4):E761-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
28. Hoppeler H, Baum O, Lurman G, Mueller M. Molecular mechanisms of muscle plasticity with exercise. Compr Physiol. 2011;1(3):1383-412. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10(8):935-45. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496-501. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Song Z, Moore DR, Hodson N, Ward C, Dent JR, O'Leary MF, et al. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep. 2017;7(1):1-14. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Si R, Tao L, Zhang HF, Yu QJ, Zhang R, Lv AL, et al. Survivin: a novel player in insulin cardioprotection against myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 2011;50(1):16-24. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Inoki K. mTOR signaling in autophagy regulation in the kidney. Semin Nephrol. 2014;34(1):2-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
34. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307-18. [View at Publisher] [DOI] [PMID] [Google Scholar]
35. Moieni A, SA Hosseini. Effect of Resistance Training Combined with Curcumin Supplementation on Expression of Regulatory Genes Related to Myocardial Remodeling in Obese Rats. Journal of Applied Health Studies in Sport Physiology. 2020;7(2):45-52. [View at Publisher] [DOI] [Google Scholar]
36. Sadeghipour HR, Raeisi F, Zar A. The effect of eight weeks of resistance training and Spirulina platensis supplementation on the signaling pathway of Wnt-GSK3β-TSC2-S6K in the kidney tissue of male rats. Journal of Applied Health Studies in Sport Physiology. 2024;11(1):223-36. [View at Publisher] [DOI] [Google Scholar]
37. Mirzaei B, Ghavami Amin H, Fadaei Chafy MR. Investigating the effect of exercise training in different periods of growth on protein synthesis (4E-BP1) and proliferation of cardiac cells (S6K1) in male rats. Journal of Exercise & Organ Cross Talk. 2023;3(3):116-23. [View at Publisher] [DOI] [Google Scholar]
38. Mousavi Mozafar SM, Nourshahi M, Akbarnejad A. Muscle Murf1 and P70S6K Before and After 6 Weeks of Resistance Training and HMB Supplementation in Inactive Men. Sport Physiology. 2020;12(45):79-94. [View at Publisher] [DOI] [Google Scholar]
39. Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB, et al. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf). 2010;199(1):71-81. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Hulmi JJ, Tannerstedt J, Selänne H, Kainulainen H, Kovanen V, Mero AA, et al. Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. Journal of applied physiology. 2009;106(5):1720-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
41. Amiri N, Amiri N, Matinhomaee H, Peeri M, Azarbaielani MA, et al. The effect of 6 weeks eeccentric training and HMB supplement on p70s6k expression of the Adult male rats. medical journal of mashhad university of medical sciences. 2019;62(5):1756-63. [View at Publisher] [DOI] [Google Scholar]
42. Phan F, Flamment M, Hu M, Hainault I, Ferré P, Foufelle F. BCAA and ER stress activate SREBP-1c cleavage and hepatic lipogenesis through mTOR. Journal of Hepatology. 2018;68(1):S339. [View at Publisher] [DOI] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






